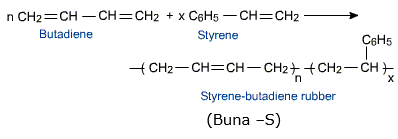Write the structures of the monomers used for getting the following polymers
(a) Nylon 6,6
(b) Melamine-formaldehyde polymer
(c) Buna-S
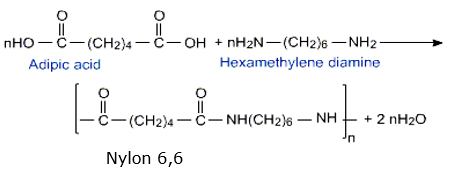
(a) Nylon 6,6 is prepared by the condensation polymerization:
Condensation polymers are formed by the repeated condensation
reaction between two bifunctional and trifunctional monomer units
usually with elimination of small molecules like water, alcohol,
ammonia, carbon dioxide, hydrogen chloride, etc. and the process
by which condensation polymers are formed is called
condensation polymerization.
Nylon 6,6 is obtained by the condensation polymerization of two
monomers, i.e., hexamethylenediamine and adipic acid, each
containing two functional groups, with the loss of water molecules.
Thus,
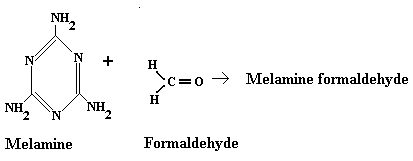
(b) Melamine Formaldehyde is also prepared by the condensation
polymerization of two monomers, i.e., Melamine and formaldehyde.
Thus,
(c) Buna-S is prepared by the addition polymerization:
Addition polymers are formed by the repeated addition of a large
number of same or different monomers possessing double and
triple bonds and the process by which addition polymers are
formed is called addition polymerization.
Buna-s is an addition polymer of two monomers, i.e., 1,3
butadiene and styrene. Thus,