The two complexes of nickel, [Ni (CN) 4]2- and [Ni (CO) 4], have different structures but possess same magnetic behavior. Explain.
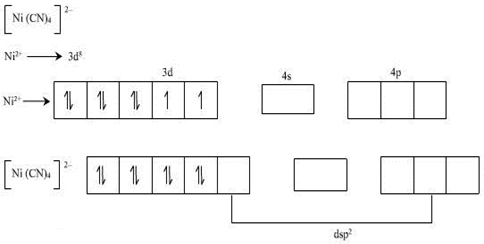
1. Let us first take the case of [Ni (CN) 4]2-.
In this complex ion the oxidation state of nickel is +2 and the electronic configuration of nickel is given by 3d8 4s2.
So the configuration of nickel [II] ion will be 3d8.
Since the ligand present in the complex ion is the cyanide group, the pairing of electrons will take place in the d-orbitals and all the unpaired electrons will get paired. Therefore the magnetic nature of this complex ion is diamagnetic as no electron is left unpaired.
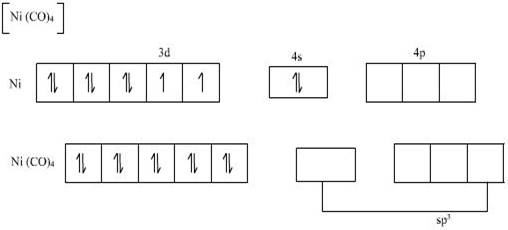
2. Let us now take the case of [Ni (CO) 4]
In this complex ion the oxidation state of nickel is zero and thus nickel is in zero oxidation state.
So the electronic configuration of Nickel in this ion is 3d8 4s2.
Here the carbonyl ligand also a strong ligand because of which pairing of electrons takes place and all the unpaired electrons gets paired up. Since no unpaired electrons remains in the orbital, this complex ion also exhibits diamagnetic property.