Write scientific reasons.
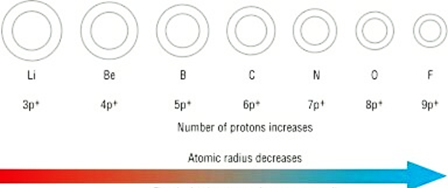
Atomic radius goes on decreasing while going from left to right in a period.
Atomic radius goes on decreasing while going from left to right in a period because of the following reasons:
⇒ Within a period, the atomic number increases one by one as a result nuclear charge increases.
⇒ The outer electrons are adding in the same valence shell.
⇒ Due to increased nuclear charge, the attraction of electrons by the nucleus increases.
⇒ Therefore, the size of the atom decreases with the increase in atomic number (number of protons).
7