Explain the structure of graphite in ten of bonding and give one property based on this structure.
Structure of graphite
i. In graphite, every carbon atom is bonded to three other carbon atoms by covalent bonds.
ii. A graphite crystal is hexagonal.
iii. The regular arrangement of atoms in graphite is in hexagonal layers.
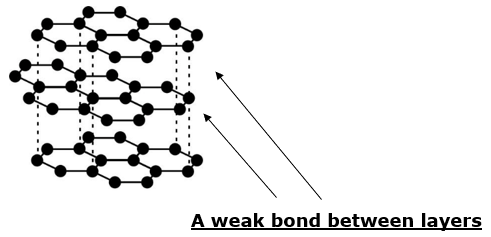
Property – A good lubricant
iv. The layers are loosely bounded with each other.
v. As there is a weak force of attraction present between them, hence the layers slide with each other.
vi. This makes the graphite slippery and thus it used as a lubricant.
24