An incomplete form of the periodic table is given below. Write answers to the questions connecting the position of elements in it.
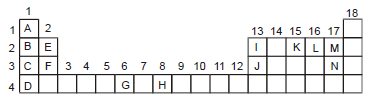
Which element has the smallest atom in period 2?
Element M
Atomic size goes on decreasing while going from left to right in a period because:
i. Within a period, the atomic number increases one by one as a result nuclear charge increases.
ii. The outer electrons are adding in the same valence shell.
iii. Due to increased nuclear charge, the attraction of electrons by the nucleus increases.
iv. Therefore, the size of the atom decreases with the increase in atomic number (number of protons).
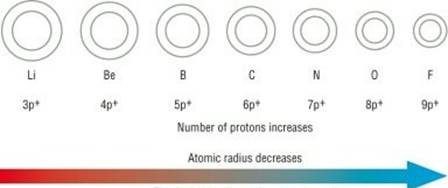
Thus, element M has smallest atom in period 2.
6