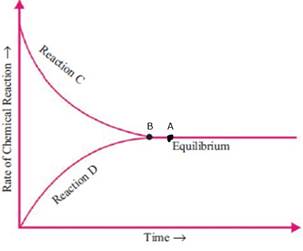
The graph for the reaction N2 (g) + 3H2 (g) ⇋ 2NH3 (g) + heat is given below.

a. Identify and write the reactions C and D.
b. What happens to the position of point A in the graph when a catalyst is used? Redraw the graph accordingly.
a) Reaction C is Forward reaction
N2 (g) + 3H2 (g) ![]() 2NH3 (g)
2NH3 (g)
Reaction D is Backward reaction
2NH3 (g) ![]() N2 (g) + 3H2 (g)
N2 (g) + 3H2 (g)
The rate of forward reaction decreases with time and the rate of backward reaction increases with time to attain the state of equilibrium.
b) When the catalyst is used then the rate of reaction will be increased and the reaction will attain equilibrium more easily. So the point A will shift towards the left side. And the new equilibrium point is B.
2