What happens when an acid or a base is added to the water? Why does the beaker appear warm? Why should we always add acid or base to the water and not water to the acid or base?
When an acid or base is added to water:
i. When we add acid to the water, acid gives H+ or H3O+ (hydronium ion) in water. For example: If we add HCl (an acid) to water, the reaction takes place is given as:
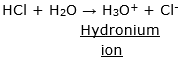
ii. When we add base to the water, base produces OH- (hydroxide ions) in water. For example: If we add NaOH to water, the reaction takes place is given as:
![]()
The beaker appears warm because the process of dissolving an acid or base in water is a highly exothermic one (releases energy)
We should always add acid or base to the water with constant stirring because if water is added to acid or base, the heat generated may cause the mixture to splash (a small blast) and cause harmful burns.