Complete the following table:

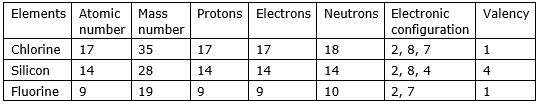
Chlorine atomic number is 17 so protons = electrons = 17; since number of protons/electrons in an atom determine the atomic number. Mass number will be sum of protons and neutrons = 17 + 18 = 35.
Since the atomic number is 17 for chlorine so electronic configuration is 2, 8, 7. Valency is 1.
Silicon atomic number is 14 = number of electrons/protons. Since the mass number is 28 so the number of neutrons is a mass number – the number of protons = 28-14 = 14.
The electronic configuration will be 2, 8, 4 and valency is 4.
Fluorine atomic number is 9 = number of protons/electrons. Mass number is 9 + 10 = 19. Electronic configuration is 2, 7 and valency 1.
13