Explain the process by which metals are obtained from its molten chloride.
Metals are obtained from their molten chlorides by the process of electrolytic reduction. Electrolytic reduction is as the name suggests reduction of metals by the process of electrolysis. Let's consider the electrolysis of fused sodium chloride:
i. Fused sodium chloride is taken as the electrolyte.
ii. Two electrodes are inserted into it.
iii. When an electric current is passed through it, then the electrolyte separates into its ions, i.e. sodium ions and chloride ions.
iv. Sodium ions migrate towards the cathode where they get reduced by gaining an electron, and thus sodium metal gets deposited at the cathode (as shown below)
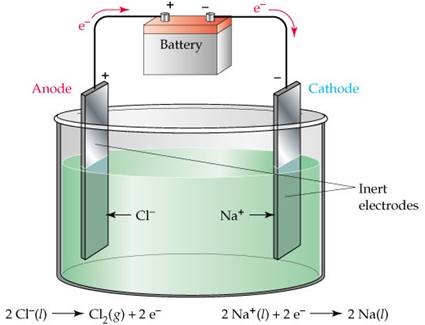
v. From there we can easily obtain sodium metal.
Note: The other highly reactive metals can be easily obtained by this process by using their respective fused salts as electrolytes.