For a certain chemical reaction variation in concentration [A] vs. time (s) plot is given below:
(i) Predict the order of the given reaction?
(ii) What does the slope of the line and intercept indicate?
(iii) What is the unit of rate constant k?

(i) Zero - order reaction
(ii) The slope of the graph represents - k, and the intercept represents [R]o
(iii) mol/l/s
Explanation:
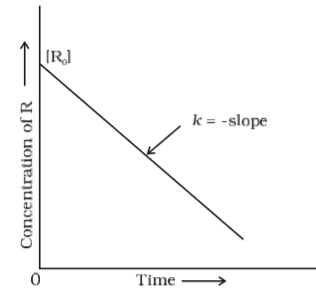
(i)In zero order reactions, the rate of the reaction is proportional to zero power of the concentration of reactants. Therefore the given reaction is a zero - order reaction.
(ii)The equation of the plot [R] = - kt + [R]o is similar to the equation of a straight line y = mx + c. Therefore comparing the two we get that slope = - k and the intercept = [R]o
10