How is Magnesium Chloride formed by the transfer of electrons? Why does the solution of Magnesium chloride conduct electricity?
Magnesium belongs to the family of Alkaline Earth Metals and hence it is the reactive metal in nature and on the other hand chlorine belongs to the halogen family and hence chlorine is also reactive in nature.
The nature of bond form between magnesium and chlorine has to be ionic as magnesium atom donates two electrons to two chlorine atoms to form magnesium chloride.
The reaction is as follows:
Mg → Mg + 2e-
Cl2 + 2e-→ 2Cl-
Hence the total reaction is given as follows:
Mg + Cl2→ MgCl2
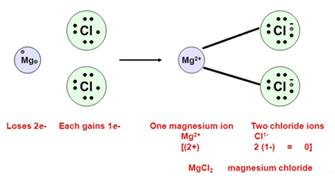
1. Magnesium Chloride is an ionic compound which means it is formed by the process of electron transfer.
2. Hence when magnesium chloride is dissolved in water or in its molten state, it will conduct electricity as ions get dissociated, and hence they conduct electricity.
3. All the ionic compound conduct electricity in their molten or aqueous state.